Regulated Studies
 ANSI/HL7 V3 ECG,R1-2004 (R2009) HL7 Version 3 Standard: Regulated Studies - Annotated ECG, Release 1 8/27/2009 |
 HL7 V3 IG ECG, R1-2004 HL7 Version 3 Implementation Guide: Annotated ECG, Release 1 2004 |
 HL7 V3 CRFQSFM R1-2008 HL7 Version 3 Standard: Regulated Studies; Clinical Research Filtered Query (CRFQ) Service Functional Model, Release 1 May 2008 |
 ANSI/HL7 V3 DSR, R2-2011 HL7 Version 3 Standard: Drug Stability Reporting (eStability), Release 2 (revision of ANSI/HL7 V3 DSR, R1-2005) 06/20/2011 |
 HL7 V3 IG DSR, R2 HL7 Version 3 Implementation Guide: Drug Stability Reporting, Release 2 February 2009 |
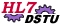 HL7 CTR&R, R1 HL7 Version 3 Standard: Clinical Trial Registration & Results, Release 1 January 2012 |
 HL7 CTR&R DAM, R1 HL7 Version 3 Domain Analysis Model: Clinical Trial Registration & Results, Release 1 May 2011 |
| Responsible Group | Regulated Clinical Research Information Management Work Group HL7 |
| Primary Contributor - CTR&R | Scott Getzin Lilly, Inc. |
| Publishing Facilitator | Becky Angeles Scenpro, Inc. |
| CTLabR2 Primary Contributor | Clinical Genomics SIG HL7 |
| RCRIM Co-Chair | Dave Iberson-Hurst CDISC |
| Primary Contributor | Barry Brown Mortara Instruments |
| Primary Contributor | CDISC Laboratory Standards Team Clinical Data Standards Interchange Consortium (CDISC) |
| Publishing Facilitator | Julie Evans Clinical Data Standards Interchange Consortium (CDISC) |
| Primary Contributor | Norman Gregory US Food and Drug Administration |
| RCRIM Co-Chair | Edward Helton National Cancel Institute |
| RCRIM Co-Chair | Randy Levin US Food and Drug Administration |
| CTLabR3 Primary Contributor | Jennifer L. Neat City of Hope |
| Primary Contributor | Phil Pochon Covance |
| RCRIM Co-Chair/Facilitator | Linda Quade Eli Lilly and Company |
| Primary Contributor - Study Participation and Study Design | Jason Rock jason.rock@globalsubmit.com GlobalSubmit |
| Primary Contributor | Gunther Schadow, M.D., PhD. Regenstrief Institute for Health Care |
| CTLabR3 Facilitator | Abdul-Malik Shakir Shakir Consulting |
| RCRIM Co-Chair | Barbara Tardiff Clinical Data Standards Interchange Consortium (CDISC) |
| RCRIM Co-Chair | Ed Tripp Independent |
| Facilitator | Mead Walker Mead Walker Consulting |
HTML Generated: 2012-08-31T12:12:25
Content Last Edited: 2012-08-06T15:01:40
HL7® Version 3 Standard, © 2004, 2008, 2009, 2011 Health Level Seven® International All Rights Reserved.
HL7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U.S. Pat & TM Off.
Use of these materials is governed by HL7 International's IP Compliance Policy.
Table of Contents
Preface
i Notes to Readers
ii Message Design Element Navigation
1 Overview
1.1 Introduction & Scope
1.2 Domain Information Models
2 AnnotatedECG Topic
2.1 Introduction
2.2 Storyboards
2.3 Application Roles
2.4 Refined Message Information Models
2.5 Hierarchical Message Descriptions
2.A Implementation Guide
3 Clinical Research Filtered Query Service Release 1 Topic
4 Drug Stability Reporting R2 Topic
4.1 Introduction
4.2 Storyboards
4.3 Application Roles
4.4 Trigger Events
4.5 Refined Message Information Models
4.6 Hierarchical Message Descriptions
4.7 Interactions
4.A Drug Stability Reporting Release 2 Implementation Guide
5 Clinical Trials Registration and Results Topic
5.1 Introduction
5.2 Storyboards
5.3 Application Roles
5.4 Trigger Events
5.5 Refined Message Information Models
5.6 Hierarchical Message Descriptions
5.7 Interactions
6 CTRR Domain Analysis Model Topic
7 Quality Analysis Report Topic
8 CMETs Defined by this Domain
9 Interactions Annex
9.1 By Application Role
9.2 By Trigger Event
9.3 By Message Type
10 Glossary
In Normative Edition 2011, the Regulated Studies domain contains two standards, two draft standards for trial use and the approved informative documents. The domain is organized under three "topics" as outlined below.
Annotated ECG topic
- Annotated ECG, Release 1 - ANSI approved standard
- Annotated ECG, Release 1 Implementation Guide - HL7 approved informative document
Clinical Research Filtered Query topic
- Clinical Research Filtered Query Service Functional Model, Release 1 - Draft Standard for Trial Use
Drug Stability Reporting topic
- Drug Stability Reporting, Release 2 - HL7 approved standard
- Drug Stability Reporting Implementation Guide - HL7 approved informative document
Clinical Trials Registration and Results topic
- Clinical Trials Registration and Results, Release 1 - Draft Standard for Trial Use
Clinical Trials Registration and Results Domain Analysis Model topic
- Clinical Trials Registration and Results Domain Analysis Model, Release 1 - HL7 approved informative document
|
||||||
|
||||||
|
This domain includes standards developed as part of the family of messages targeted for the exchange of information about the conduct of regulated studies, and the exchange of the data collected during those studies. This family includes, but is not limited to, standards for submission of clinical trial and product stability information and data to a regulatory agency.
The principal contributor to this domain is the Regulated Clinical Research Information Management (RCRIM) technical committee.
This committee supports the HL7 mission to create and promote its standards by developing standards to improve or enhance information management during research and regulatory evaluation of the safety and efficacy of therapeutic products or procedures worldwide. The committee defines messages, document structures, and terminology to support the systems and processes used in the collection, storage, distribution, integration and analysis of such information. The work of this committee will facilitate the availability of safe and effective therapies by improving the processes and efficiencies associated with regulated clinical research.
SCOPE
The Regulated Studies domain currently contains specifications addressing Product Stability Reporting (to a national regulatory agency), Annotation of ECGs (for submission to a drug regulatory agency); and the Periodic Reporting of Clinical Trial Laboratory Results (to a study sponsor or sponsor's agent).
Summary of Clinical Trial Interactions in the Regulated Studies Domain
An Interaction is the formatted package of data to be exchanged between a named sender and a named receiver in response to a named trigger event.
The diagram above provides a context for understanding all clinical trial interactions in Regulated Studies. Each storyboard will be accompanied with a similar diagram showing only the interactions described in that storyboard.
The large gray boxes represent potential application systems that might be involved in the exchange of Regulated Studies information. There is no expectation that these application systems must reside at different physical locations. Nor need they be under the control of different parties. All application systems could, for example, be present at and managed by the sponsor. Alternatively, they could be distributed among several parties and locations.
The smaller boxes within represent system functionality that should be present in the application system to handle the exchange. These application system functionalities are called Application Roles.
The horizontal arrows represent the actual interchange of information between application roles, using the messages in this domain.
This interaction diagram shows the four clinical trial interactions currently included in this domain.
Also shown are four potential future interactions -- a request for ECG analysis from the study site; a request for ECG analysis from the Sponsor/Agent; a request for laboratory analysis from the study site; and a report of laboratory results from the laboratory to the study site.
| Return to top of page |