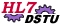 HL7 CTR&R, R1 HL7 Version 3 Standard: Clinical Trial Registration & Results, Release 1 January 2012 |
Content Last Edited: 2012-08-06T15:01:40
5.2 Storyboards
5.3 Application Roles
5.4 Trigger Events
5.5 Refined Message Information Models
5.6 Hierarchical Message Descriptions
5.7 Interactions
This is a Draft Standard for Trial Use of the Version 3 Clinical Trial Registration and Results (CTR&R), Release 1 topic. This topic, in the Regulated Studies domain, was approved as a DSTU following a successful DSTU ballot during HL7 International's May 2010 ballot cycle.
"Publication of this draft standard for trial use and comment has been approved by Health Level Seven, Inc. (HL7). This draft standard is not an accredited American National Standard. The comment period for use of this draft standard shall end 12 months from the date of publication. Suggestions for revision should be submitted at //www.hl7.org/dstucomments/index.cfm.
Following this 12 month evaluation period, this draft standard, revised as necessary, will be submitted to a normative ballot in preparation for approval by ANSI as an American National Standard. Implementations of this draft standard shall be viable throughout the normative ballot process and for up to six months after publication of the relevant normative standard
The Clinical Trial Registration message is a message specification for an interaction containing a clinical trial protocol specification between a clinical trial protocol authoring organization and a registration authority and for interaction between clinical trial registration authorities.
|
||||||
|
For details on the interpretation of this section, see the storyboard discussion in the Version 3 Guide.
The Storyboard is presented as a high-level description of the business situation the standard is designed to address, accompanied by a diagram of the interactions that will be described in the narratives that follow. Interactions were discussed in the Introduction and Scope section of the Regulated Studies domain chapter, under the heading "Summary of Clinical Trial Interactions in the Regulated Studies Domain". There you will find a diagram of the AnnotatedECG and Clinical Trial laboratory interactions in context.
A number of narrative sections follow each high-level description, each describing a scenario in which one of the interactions might be used. These narratives are for illustrative purposes only. They are not meant to limit the applicability of the standard, nor to proscribe minimum requirements for using the standard. In particular, there is no intent to establish regulatory agency policy on management of data or processes through these scenarios. Such policy will be separately published by the regulatory agencies (eg. as Implementation Guides or Guidances).
The clinical trial registration process includes a submission of a clinical trial protocol specification to a registration authority. This same payload structure may also be useful for data exchange from one registry authority to another or from a sponsoring organization to a ethics review body; however, these specific alternate use cases have not been fully explored.
Only the Clinical Trial Registration submission from the sponsoring organization to the registration authority is being balloted at this time.
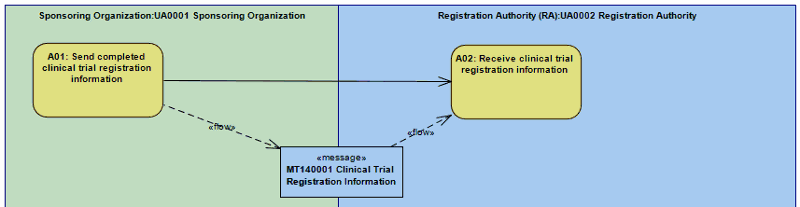
| Clinical Trial Registration |
A Sponsoring Organization submits Clinical Trial Registration Information to a Registration Authority.
|
||||||||
|
For details on the interpretation of this section, see the discussion of application roles and their relationships in the Version 3 Guide.
|
||||||
|
For details on the interpretation of this section, see the discussion of trigger events in the Version 3 Guide.
|
||||||
|
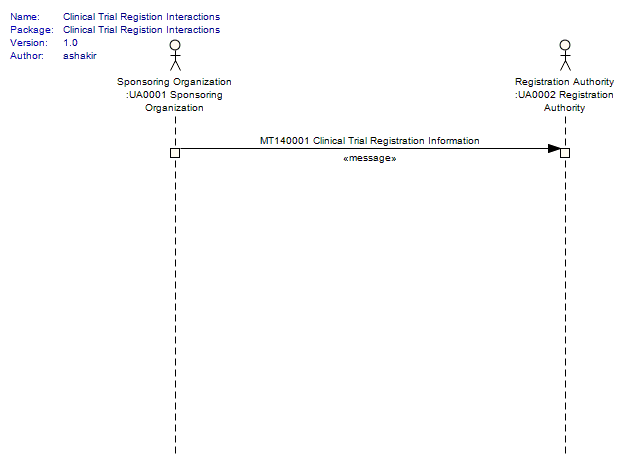
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
| Parent: | None |
| Clinical Trial Registration | PORT_HD140001UV01 |
|
||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
The clinical trial registration HMD is the serialized version of the RMIM used to model the payload included in a clinical trial registration interaction. It contains protocol data describing the study in sufficient detail to enable assessment and registration by the registration authority.
| Clinicaltrialsregistry Message Type | PORT_MT140001UV01 |
|
||||||
|
For details on the interpretation of this section, see the definition of Interactions in the Version 3 Guide.
An Interaction is the formatted package of data to be exchanged between a named sender and a named receiver in response to a named trigger event.
Submission of an protocol specification for an intended study. Submitted by the study sponsoring organization to a registration authority.
| Trigger Event | Clinical Trial Registration Submission | PORT_TE140001UV01 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Clinicaltrialsregistry Message Type | PORT_MT140001UV01 |
| Sender | Sponsor Organization | PORT_AR140001UV01 |
| Receiver | Registration Authority | PORT_AR140002UV01 |
| Return to top of page |

