|
||||||||
|
| Parent: | None |
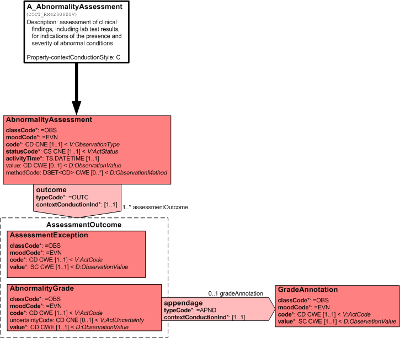
The AbnormalityAssessment CMET is used to communicate data about preliminary, first-level assessments of clinical findings, including laboratory test results, for indications of the presence and severity of abnormal conditions.
Overview
The interactions currently supported by this CMET include:
- CT Lab Test Result Abnormality Assessment Request
- CT Lab Test Result Abnormality Assessment Response
Abnormality Assessment (entry point): The process of evaluating a clinical finding, including a laboratory test result, for indications of the presence and severity of an abnormal condition, and to assign the corresponding descriptive terminology that identifies the abnormal condition.
Assessment Exception: An exception represents one of two possible outcomes of the abnormality assessment (Abnormality Grade being the other possible outcome). When an assessment can not be done, information is provided detailing the reason(s) for the exception(s).
Abnormality Grade: An abnormality grade represents one of two possible outcomes of the abnormality assessment (Assessment Exception being the other possible outcome). When an assessment is completed, the severity of the abnormal condition is provided and this grade/level of severity is based on a specified assessment criteria or scale.
GradeAnnotation: Additional information provided about a grade such as explanatory text, caveats, and constraints.
| A_AbnormalityAssessment universal | COCT_MT420000UV |
| Parent: | None |
This CMET is entered through the SubjectAssignement class, which represents enrollment in the study at a particular site. Component relationships are used to show the association of the subject enrollment to the StudyAtSite class and the site study to the overall ResearchStudy class. The following describes the individual associations to these three central classes.
TreatmentGroupAssignemnt: The definition act-relationship is used to show when the subject is also assigned to a treatment arm. This class is in Definition mood because a treatment arm is a treatment definition for a subgroup of the total enrollment.
ResearchOrganizaton and Site: The location participation captures the research institution responsible for the study at a particular site.
The ApprovedResearchOrganization represents the role of the research institution as an approved institution for federally-funded research as indicated by the Assurance number issued by the Office of Human Subject Research Protection under HHS.
IRBChair: The verification participation is used to identify the Institution Review Board and its chair. The IRB is responsible for ensuring the ethical conduct of the study at a given site with respect to the protection of human subjects.
SiteInvestigator and StudyInvestigator: The performer participation captures the lead for the study at a particular site as identified in the SiteInvestigator class and the lead investigator for the whole study is identified through the responsible party participation in the StudyInvestigator class. In addition, the department where the lead investigator works is identified in the Department class.
StudyInformation: The pertains to act-relationship relates the ResearchStudy class and the StudyAtSite class to the StudyInformation class to identify observations about the study. For example, any action that may be taken to the study as a result of the event or various statistics about enrollments.
StudyProtocol: The instantiation act-relationship associates the StudyProtocol class to the research study. The protocol is the overall plan for conducting the study. It must first be authorized by a regulatory organization before the study can take place. ProtocolAuthorization contains the authorization number and type of authorization, for example IND for drugs or IDE for devices. The Country class identifies the country where the regulatory organization issued the authorization.
ContractAward: The authorization act-relationship connects the ContractAward class to the study. In federally-funded research, the study is usually performed by the research institution under a contract to either the sponsor or the funding organization. Both the contract number and the type of contract are identified in the ContractAward class.
ResearchSponsor: The sponsor for the research is identified through the author participation in the ResearchSponsor class. The sponsor is the organization that proposed the research.
IdentifiedResearchStudy: The reference act-relationship is used to associate multiple identifiers with the study. The IdentifiedResearchStudy contains the identifier and the Agent class contains the type of organization that assigned the identifier.
| A_ResearchSubjectEnrollment | COCT_MT970000UV |
| Return to top of page |

