 ANSI/HL7 V3 DSR, R2-2011 HL7 Version 3 Standard: Drug Stability Reporting (eStability), Release 2 (revision of ANSI/HL7 V3 DSR, R1-2005) 06/20/2011 |
 HL7 V3 IG DSR, R2 HL7 Version 3 Implementation Guide: Drug Stability Reporting, Release 2 February 2009 |
Content Last Edited: 2012-08-06T15:01:40
4.2 Storyboards
4.3 Application Roles
4.4 Trigger Events
4.5 Refined Message Information Models
4.6 Hierarchical Message Descriptions
4.7 Interactions
4.A Drug Stability Reporting Release 2 Implementation Guide
Release 2 of the stability message (including material for drug stability testing) passed its HL7 Normative ballot following the HL7 May 2010 ballot cycle. The specification is designed to capture the contents of a stability report to be submitted to a national regulatory agency. At this point, the primary point of reference is current stability reporting within the United States. The scope of a single transaction or message in this specification has been restricted to only include studies with a single set of storage conditions. However, the stability studies that are filed with regulatory authorities are not simply a study with a single set of storage conditions - the studies include a number of storage conditions plus multiple orientations (upright and inverted). This is especially true for injectable/Parenteral products. These more complex situations, that is to say, studies that require multiple storage conditions and or orientations (e.g., accelerated, room temperature, inverted, upright, etc.), will be handled through the submission of multiple linked reports, with each report covering a single storage condition and orientation.
The changes from the initial DSTU to this normative ballot are as follows: 1) Added the attribute "text: ED [0..1]" to the AssociatedStudy class, 2) Added the attribute "expirationTime*: IVL_TS [1..1]" to Substance class; and 3) Changed the cardinality of component5 going from the class StabilityStudy to the class StudyOnBatch from"1..* studyOnBatch*" to 1..1 studyOnBatch*".
The changes from R1 to Release 2 DSTU (now Normative) are as follows: 1) Added AssociatedStudy with an order (sequenceNumber) so stability reports can be linked and in a specific order if needed, 2) Changed the name Organization to StudySponser, 3) Made organization ID zero to many (StudySponser, Manufacturer, TestingSite, and SiteStub) to allow an organization to be identified by many systems (e.g., OID, FEI, DUNS, etc.), 4) Linked Storage directly to StudyOnBatch eliminating the prior choice box holding Storage and Testing because only a single set of storage conditions is supported, and 5) Moved TestingSite under Testing with zero to many and added SiteStub under Test so the site performing the test may be identified by ID number reducing the redundancy of information for Test.
|
||||||
|
For details on the interpretation of this section, see the storyboard discussion in the Version 3 Guide.
The Storyboard is presented as a high-level description of the business situation the standard is designed to address, accompanied by a diagram of the interactions that will be described in the narratives that follow. Interactions were discussed in the Introduction and Scope section of the Regulated Studies domain chapter, under the heading "Summary of Clinical Trial Interactions in the Regulated Studies Domain". There you will find a diagram of the AnnotatedECG and Clinical Trial laboratory interactions in context.
A number of narrative sections follow each high-level description, each describing a scenario in which one of the interactions might be used. These narratives are for illustrative purposes only. They are not meant to limit the applicability of the standard, nor to proscribe minimum requirements for using the standard. In particular, there is no intent to establish regulatory agency policy on management of data or processes through these scenarios. Such policy will be separately published by the regulatory agencies (eg. as Implementation Guides or Guidances).
The storyboard provides an example of a situation in which the Public Health Stability Reporting message would be used.
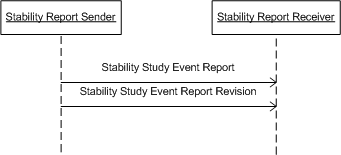
| Stability Study Event Report | |
| Stability Study Event Report Revision |
Interactions: Stability Report (PORT_IN090001)
ChemCentric Drug Company has been working on a new product, CureAll. Development work on the product has been completed, as have many aspects of the regulatory process. One aspect of the regulatory process is to demonstrate the stability of the CureAll product by testing it against an established Testing Specification using an acceptable protocol. ChemCentric has carried out the necessary testing, and reports the test results to the applicable regulatory authority.
Interactions: Stability Report Revision (PORT_IN090002)
Last month, ChemCentric Drug Company submitted a stability study for its new product, CureAll, to the FDA
However, Dr. Reggie Review has been going over the results of the test. He has discovered a transcription error that could lead reviewers to get an unfair estimation of CureAll's efficacy even after lengthy storage. In order to correct this state of affairs, ChemCentric submits a revised Stability Study.
|
||||||||
|
For details on the interpretation of this section, see the discussion of application roles and their relationships in the Version 3 Guide.
|
||||||||
|
For details on the interpretation of this section, see the discussion of trigger events in the Version 3 Guide.
|
||||||
|
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
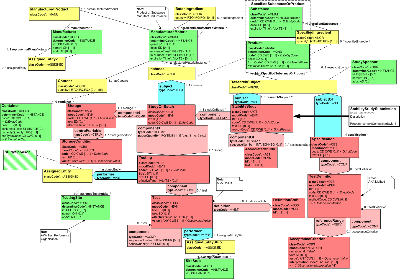
| Parent: | None |
The Drug Stability Report RMIM captures information relevant for the drug stability testing process. This testing is required in the United States as a component of the drug regulatory process. It verifies the correctness of a manufacturer's claims related to the stability - the ability to be stored over time without losing its therapeutic effectiveness - of a product.
It is important to note that the material that can be reported as part of a Stability Study is not limited to the items explicitly listed as attributes within the RMIM. This is because the "text' attribute in Acts (shown in red on the diagram), and the "desc" attribute in Entities (shown in green on the diagram) can be valued with a URL which provides a link to additional documentation. This additional documentation would either be provided along with the stability report, or would be stored at the sender's site at the location indicated by the URL.
The HMD file provides a discussion of the classes, associations, and attributes within the model.
| StabilityStudySubmission | PORT_HD090002UV01 |
|
||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
| Stability Study Message Type | PORT_MT090002UV01 |
|
||||||||
|
For details on the interpretation of this section, see the definition of Interactions in the Version 3 Guide.
An Interaction is the formatted package of data to be exchanged between a named sender and a named receiver in response to a named trigger event.
The interaction supports revisions to previously sent stability messages.
| Trigger Event | Stability Report Revise Notification | PORT_TE090002UV01 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Stability Study Message Type | PORT_MT090002UV01 |
| Sender | Stability Report Sender | PORT_AR090001UV01 |
| Receiver | Stability Report Receiver | PORT_AR090002UV01 |
The interaction supports the communication of a new stability report
| Trigger Event | Stability Report Notification | PORT_TE090001UV01 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Stability Study Message Type | PORT_MT090002UV01 |
| Sender | Stability Report Sender | PORT_AR090001UV01 |
| Receiver | Stability Report Receiver | PORT_AR090002UV01 |
These Implementation Guides (IGs) are for the use of the Health Level 7 (HL7) Stability Standard. Each IG describes the basic requirements needed for using the standard and the requirements needed for submitting information to the Food and Drug Administration (FDA) using the standard. This standard is meant to be used for all regulated products (e.g., test kits, drugs, reagents, medicated feeds, etc.), excluding foods.
The HL7 Stability Standard is for one way communication between contractor to company, company to company, company to regulatory agency.
Available Implementaton Guides:
- HL7 Version 3 Implementation Guide: Drug Stability Reporting (eReporting) R2, Release 3 (2012 February)
- HL7 Version 3 Implementation Guide: Drug Stability Reporting (eReporting) R2, Release 2 (2010 November)
- HL7 Version 3 Implementation Guide: Drug Stability Reporting (eReporting) R1, Release 2 (2009 February)
| Return to top of page |

