 HL7 CCPM, R1 HL7 Version 3 Standard: Common Product Model CMETs, DSTU Release 11 June 2011 |
Content Last Edited: 2011-06-14T14:26:17
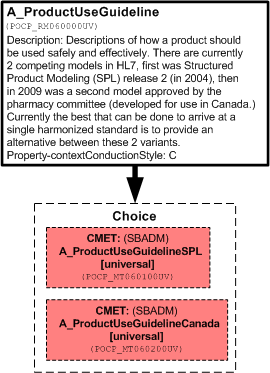
Descriptions of how a product should be used safely and effectively.
There are currently 2 competing models in HL7, first was Structured Product Modeling (SPL) release 2 (in 2004), then in 2009 was a second model approved by the pharmacy committee (spearheaded for use in Canada.) Currently the best that can be done to arrive at a single harmonized standard is to provide an alternative between these 2 variants.
|
||||||||||
|
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
| Parent: | ProductKind (POCP_DM010000UV) |
| A_ProductUseGuidelineCanada | POCP_HD060200UV01 |
| Parent: | ProductKind (POCP_DM010000UV) |
Describes when and how a product should be used safely and effectively. Contains the dosing instructions, indication, issues (adverse effects, interactions, contraindications), and a safe use protocol which includes monitoring observations.
| A_ProductUseGuidelineSPL | POCP_HD060100UV01 |
| Parent: | ProductKind (POCP_DM010000UV) |
Descriptions of how a product should be used safely and effectively.
There are currently 2 competing models in HL7, first was Structured Product Modeling (SPL) release 2 (in 2004), then in 2009 was a second model approved by the pharmacy committee (spearheaded for use in Canada.) Currently the best that can be done to arrive at a single harmonized standard is to provide an alternative between these 2 variants.
| A_ProductUseGuideline | POCP_HD060000UV01 |
|
||||||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
| A_IndicationBasic | COCT_MT980050UV |
| A_SubstanceAdministrationRequestUniversal | PORX_MT980040UV |
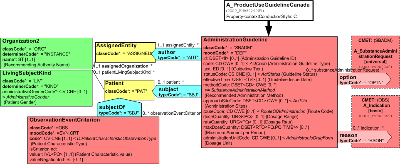
| ProductUseGuideline(Canada) | POCP_MT060200UV01 |
Describes when and how a product should be used safely and effectively. Contains the dosing instructions, indication, issues (adverse effects, interactions, contraindications), and a safe use protocol which includes monitoring observations.
| E_ProductMaterialKindUniversal | POCP_MT010400UV01 |
| R_ProductUseExactUniversal | POCP_MT010600UV01 |
| A_ProductCharacteristicUniversal | POCP_MT050200UV01 |
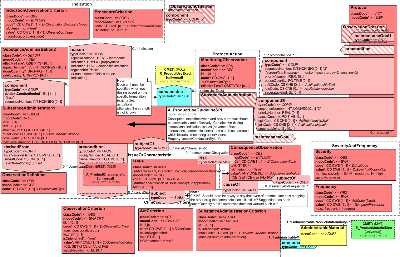
| ProductUseGuideline(SPL) | POCP_MT060100UV01 |
Descriptions of how a product should be used safely and effectively.
There are currently 2 competing models in HL7, first was Structured Product Modeling (SPL) release 2 (in 2004), then in 2009 was a second model approved by the pharmacy committee (spearheaded for use in Canada.) Currently the best that can be done to arrive at a single harmonized standard is to provide an alternative between these 2 variants.
| A_ProductUseGuidelineSPLUniversal | POCP_MT060100UV01 |
| A_ProductUseGuidelineCanadaUniversal | POCP_MT060200UV01 |
| A_ProductUseGuideline | POCP_MT060000UV01 |
| Return to top of page |

