 ANSI/HL7 V3 IDC, R1-2006 HL7 Version 3 Standard: Implantable Device Cardiac - Follow-up Summary, Release 1 11/2/2006 |
Content Last Edited: 2008-06-04T10:51:25
2.2 Storyboards
2.3 Application Roles
2.4 Trigger Events
2.5 Refined Message Information Models
2.6 Hierarchical Message Descriptions
2.7 Interactions
The Implantable Cardiac Device topic contains models, messages, and other artifacts that are needed to support messaging related to the life of an implanted cardiac device. These messages include: registration and association of implanted device with a patient; patient observation and episode information; current device therapy settings and adjustments of device therapy settings; device diagnostics; and tracking a device throughout its life.
Implanted Cardiac Devices operate within the human body in both monitoring and therapy delivery capacities. These devices are categorized as Pacemakers, Implantable Cardioverter Defibrillators (ICDs), and Cardiac Resynchronization Therapy (CRT) devices. Each of these electronic devices delivers a controlled electric shock to the heart to return the heart back to a normal rhythm.
This topic is focused on developing messages to facilitate in the transfer of implanted cardiac device data:
Device Identification Data
-
Manufacturer
-
Model
-
Serial Number
Device Therapy Settings
-
Pacing settings
-
Defibrillation settings
-
Therapy tuning features
-
Arrhythmia Logging Data
Diagnostic Data
-
Trended diagnostics on device functionality (e.g., lead impedance, pacing thresholds)
-
Diagnostic data for titration of patient therapy
Data from device follow-up testing
-
Inductions
-
Various device status tests
The IDC project team plans to develop normative terminology and messaging standards for implantable cardiac devices to facilitate their integration into health care systems, electronic medical records, and a variety of applications.
|
||||||
|
For details on the interpretation of this section, see the storyboard discussion in the Version 3 Guide.
The purpose of this storyboard is to illustrate the messaging related to the clinical review of implantable cardiac device status
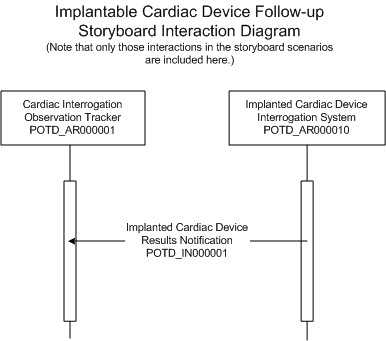
| Implanted Cardiac Device Results Notification |
Adam Everyman presents at the electrophysiology (EP) follow-up clinic for his appointment. Adam will present for follow-up 7-10 days after implant and every 3-6 months thereafter, depending on the therapy protocol.
Dr. Ed Electrode, an Electrophysiologist - also referred to as a following physician, and Nancy Nightingale, an R.N., work in the electrophysiology (EP) follow-up clinic.
Note: In the area of Electrophysiology, a "programmer" is a commonly used term to describe a specialized computer that is capable of communicating with an implanted device. Programmers are used to interrogate implanted devices and "program", or make changes to, implanted cardiac device settings.
Nancy places a wand over the implant area. The wand is connected to a programmer that interrogates the device and extracts the data (e.g., settings, status, events) from the device. Nancy reviews the device data and captures the "current state" device data from the programmer screen and/or prints out the settings and/or uses an information transfer mechanism (e.g., floppy disk, analog cable, etc.) to transmit device data, which is in a proprietary format, to a translator system.
If the device data has been sent to the translator system, the clinician may desire to transmit data to the electronic health record (EHR). In this case, a necessary subset (pre-determined by the clinic and the entity responsible for the translator system) of the data that represents the device's 'summary data' is converted from the proprietary format and transmitted using HL7 messaging to the EP office electronic health record system (EHR). This summary data is considered to be a 'snapshot' of the device at a moment in time.
Dr. Ed Electrode reviews the device data and identifies appropriate changes to device settings. Nancy Nightingale makes these changes via the programmer. Nancy then captures the "final state" device data. Nancy then uses the information transfer mechanism to transmit device data, which is in a proprietary format, to a translator system, which again converts the data into an HL7 message and communicates the 'summary data' to the clinic's EHR. This second message will utilize the same HL7 message format as the first.
Note: In this storyboard the "initial state" and "final state"' are really subjective titles for device data at a moment in time. Therefore, there is no need to make a distinction between these two "states" in the message model.
It is important to note that a clinician can initiate one or two HL7 transmissions to the clinic's EHR. This choice is based on a given clinic's workflow. In either case the interaction is the same.
These reports are sent as unsolicited observation events.
A device summary report contains the following items:
-
Device Diagnostics - represented by the DeviceTherapy act in the message model
-
Events Counters - represented by the PatientObservation act in the message model
-
Device Observations (except electrocardiograms) - represented by the DeviceObservation act in the message model
-
Programmed Therapy Settings - represented by the DeviceTherapySetting act in the message model
-
Clinician Comments - represented by the PatientObservation act in the message model
Portions of the previous storyboard may also apply to Adam Everyman having his device followed remotely. Adam will present to a device located outside of the clinic (e.g., in Adams residence) which will capture the state of his implanted device and will transmit the information to a translator system. The translator system, converts the data into an HL7 message and communicates the 'summary data' to the clinic's EHR.
|
||||||||
|
For details on the interpretation of this section, see the discussion of application roles and their relationships in the Version 3 Guide.
An implantable cardiac device interrogation system acquires information that an implantable device has captured about itself, about the patient, and about the therapies that the device has delivered. This system then translates the data from the implantable device format and communicates it to clinical information systems
|
||||||
|
For details on the interpretation of this section, see the discussion of trigger events in the Version 3 Guide.
|
||||||
|
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
| Parent: | Implantable Cardiac Device (POTD_DM000000UV) |
See DMIM description for details about this message.
| Implanted Cardiac Device Follow-Up | POTD_HD000001UV02 |
|
||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
Message types based upon this HMD are to be used to communicate for Cardiac Device follow-up
| A_Encounter | COCT_MT010000UV01 |
| R_Patient | COCT_MT050002UV07 |
| R_AssignedEntity | COCT_MT090002UV01 |
| R_AssignedDevice | COCT_MT090300UV01 |
| Implanted Cardiac Device Event New | POTD_MT000001UV02 |
|
||||||
|
For details on the interpretation of this section, see the definition of Interactions in the Version 3 Guide.
This interaction is used by the Implanted Device interrogation system to notify the receiving clinical information system of the observation results from the interrogation.
| Trigger Event | Implanted Cardiac Device Follow-Up | POTD_TE000001UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Implanted Cardiac Device Event New | POTD_MT000001UV02 |
| Reason | Trigger Event | Interaction |
| POTD_TE000001UV02 | POTD_IN000001UV02 |
| Sender | Implanted Cardiac Device Interrogation System | POTD_AR000010UV02 |
| Receiver | Cardiac Interrogation Observation Tracker | POTD_AR000001UV02 |
| Return to top of page |

