Therapeutic Devices
 ANSI/HL7 V3 IDC, R1-2006 HL7 Version 3 Standard: Implantable Device Cardiac - Follow-up Summary, Release 1 11/2/2006 |
| Responsible Group | Orders and Observations Work Group HL7 |
| Orders & Observations Co-Chair | Hans Buitendijk Siemens Medical Solutions Health Services |
| Orders & Observations Co-Chair | Gunther Schadow, M.D., PhD. Regenstrief Institute for Health Care |
| Orders & Observations Co-Chair | Jeff Sutherland PatientKeeper, Inc. |
| Implantable Device - Cardiac (IDC) Project Manager | Doug Baird Guidant |
| Implantable Device - Cardiac (IDC) Project Leader | Linda Halvorson Guidant |
| Content Contributor | David Marotz Accenture |
| Content Contributor | Pete Palmer |
HTML Generated: 2012-08-31T12:13:21
Content Last Edited: 2008-06-04T10:51:25
HL7® Version 3 Standard, © 2006 Health Level Seven® International All Rights Reserved.
HL7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U.S. Pat & TM Off.
Use of these materials is governed by HL7 International's IP Compliance Policy.
Table of Contents
Preface
i Notes to Readers
ii Message Design Element Navigation
1 Overview
1.1 Introduction & Scope
1.2 Domain Information Models
2 Implantable Cardiac Device Topic
2.1 Introduction
2.2 Storyboards
2.3 Application Roles
2.4 Trigger Events
2.5 Refined Message Information Models
2.6 Hierarchical Message Descriptions
2.7 Interactions
3 Quality Analysis Report Topic
4 CMETs Used by this Domain
5 Interactions Annex
5.1 By Application Role
5.2 By Trigger Event
5.3 By Message Type
6 Glossary
Therapeutic Devices Domain Notes to Reader
The contents of this domain have been created by the "Implantable Device - Cardiac" Project. The current focus has been on messaging surrounding implantable cardiac devices, including pacemakers, implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices, and implantable sensors. Pacemakers, IDCs, and CRT devices are used to provide therapies to people with appropriately indicated heart conditions. In addition to providing therapy, these devices and other devices within this domain capture information about the patient, the device, and therapies delivered by the device. This information is used for the observations that the message model supports.
The DMIM and associated RMIM for this domain were developed under the guidance of the Orders and Observations Technical Committee. To develop the DMIM and associated RMIM, HL7 members provided input that focused on the extensibility of the models to related areas (e.g., non-cardiac implantable devices, at-home follow-up of implantable devices, etc.).
As the Therapeutic Devices domain evolves, we anticipate the development of additional RMIMs that will be introduced by groups interested in areas outside of implantable cardiac devices.
The primary participants in the Implantable Cardiac Device industry have provided input such that the current DMIM and associated RMIM supports clinical messaging requirements for the industry.
|
Therapeutic Devices Domain
The therapeutic devices domain comprises the models, messages, and other artifacts that are needed to support messaging related to therapy delivery and observations made by a medical device.
Currently, the scope has been focused only on implantable cardiac devices (pacemakers, defibrillators, etc.).
Diagram
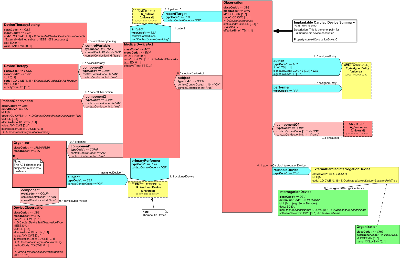
Domain Message Information Models (D-MIM) Therapeutic Devices (POTD_DM000000) Overview
The Therapeutic Devices domain comprises the models, messages, and other artifacts that are needed to support messaging related to therapy delivery and observations made by therapeutic medical devices.
The Therapeutic Devices D-MIM is a refined subset of the HL7 Reference Information Model (RIM) that includes the set of classes, attributes and relationships used to create messages for reporting information captured from these devices about patients and the device themselves.
Currently, the scope has been focused only on implantable devices (pacemakers, defibrillators, etc.), which explains the simple nature of this message model. However, over time, it is anticipated that this D-MIM will expand to support more device types and device messaging systems.
This walk-through examines the DMIM from two perspectives, the observed (the patient and their device), and the observer (the care giver, associated personnel, and the device used to interrogate the patient's device).
Focal Classes The model is focused on the Observation class. For therapeutic devices, such Observations are also known as "interrogations"; however, this model was designed such that it will be applicable to a wide range of implantable therapeutic devices. This Observation leverages the MedicalDeviceAct class for Observation detail concerning the device settings, delivered therapies, as well as patient and device statuses.
D-MIM Core Structure The core structure of the model is composed of the Observation act in the event mood. This core structure indicates how interrogations and observations are related, see figure:
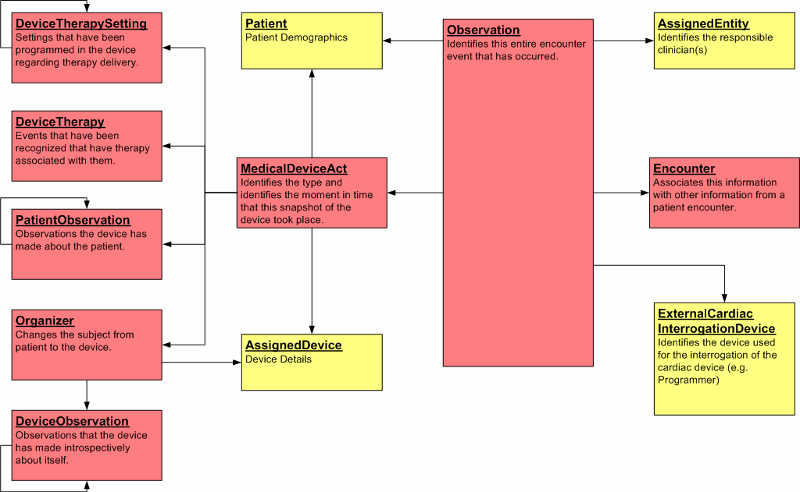
Therapeutic Devices Domain Information Model Classes Observation This D-MIM focuses on the Observation-Act of a device interrogation. All the other classes shown are only interesting and relevant in relationship to this Observation-Act. The relevant information items, attributes, and associations have a consistent meaning for observation definitions for device interrogations.
The reader should refer to the documentation for the HL7 Reference Information Model for a complete set of attribute descriptions.
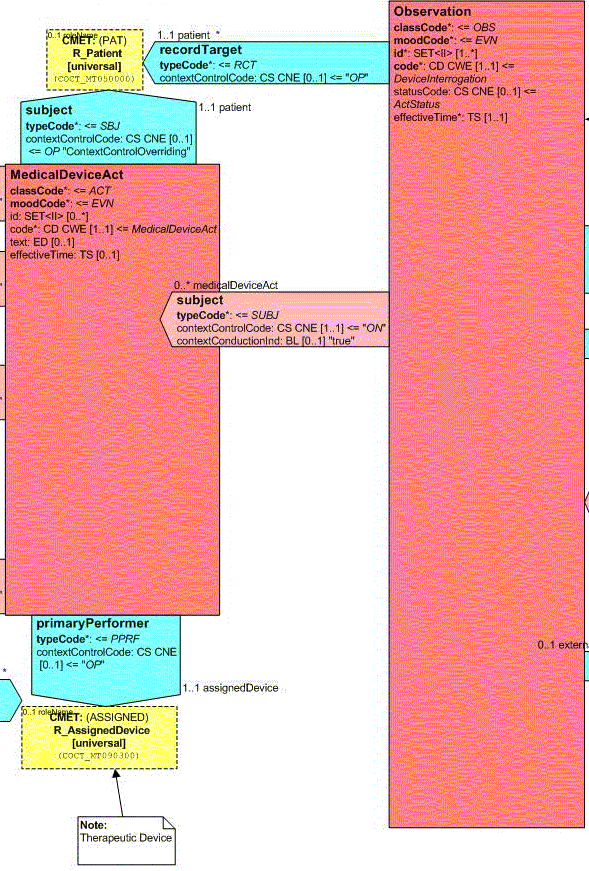
Participations and Act Relationships
The following associations are defined for a therapeutic device observation. Each association describes the relationship between the observation act and an entity or act that plays a significant part in the observation event.
Observed
From the Observed From the observed vantage point, the subject Act Relationship is involved in instantiating an observation with a Participation of recordTarget. The subject Act Relationship from the Observation act to the MedicalDeviceAct is only applicable when there is a patient bound to the particular device, as shown in this Figure:
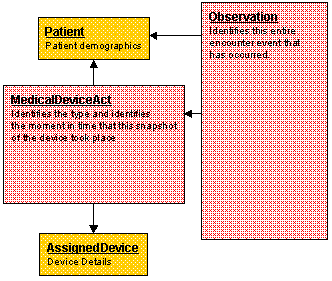
-
Observation - Represents the observation using a mandatory unique identifier and time stamp.
-
Patient - Contains patient demographics consisting of First Name, Last name, Middle Initial, Date of Birth, identification number, and gender. Patient attributes are defined in the R_Patient Common Message Element Type (CMET).
-
Medical Device Act (MedicalDeviceAct) - Describes the device interrogation that is performed (e.g., At Home device interrogation, In Office device interrogation, etc.)
-
Device - Details the therapeutic device being observed (e.g. Device Name, Device Model, Device Serial Number, Device Manufacturer). Device attributes are defined in the R_AssignedDevice CMET.
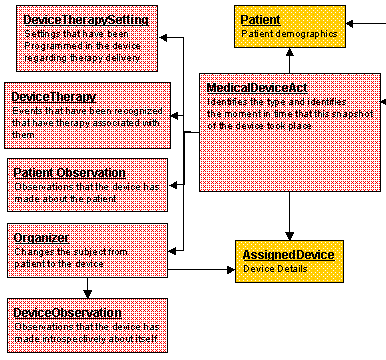
Device and Patient Observation ActsThe Device and Patient Observations are:
-
Device Therapy Settings: The settings in a device that relate to delivering therapies to the Patient (e.g., setting type, setting value)
-
Device Therapy: The events that are captured when a device delivers a therapy to the Patient (e.g., date/time of event, event description)
-
Patients Observations: The observations that a Device has made about the owning patient (e.g., observation type, observation value)
-
Organizer: This Act changes the subject from patient to device
-
Device Observations: The observations that a Device has made about itself (e.g., observation type, observation value)
The following Figure describes the device and patient observations:
 Medical Device Act Relationships
The Medical Device Act has the following Act Relationships for associating the device and patient message components with
the Observation:
Medical Device Act Relationships
The Medical Device Act has the following Act Relationships for associating the device and patient message components with
the Observation:
-
controlVariable - associates the Device Therapy Settings with the Medical Device Act
-
component1 - associates the Organizer Act with the Medical Device Act
-
component2 - associates Patient Observations with the Medical Device Act
-
component3 - associates Device Therapy with the Medical Device Act
Observer
From the
observer
vantage point, there are three Participations in the Observation as shown in the following Figure:
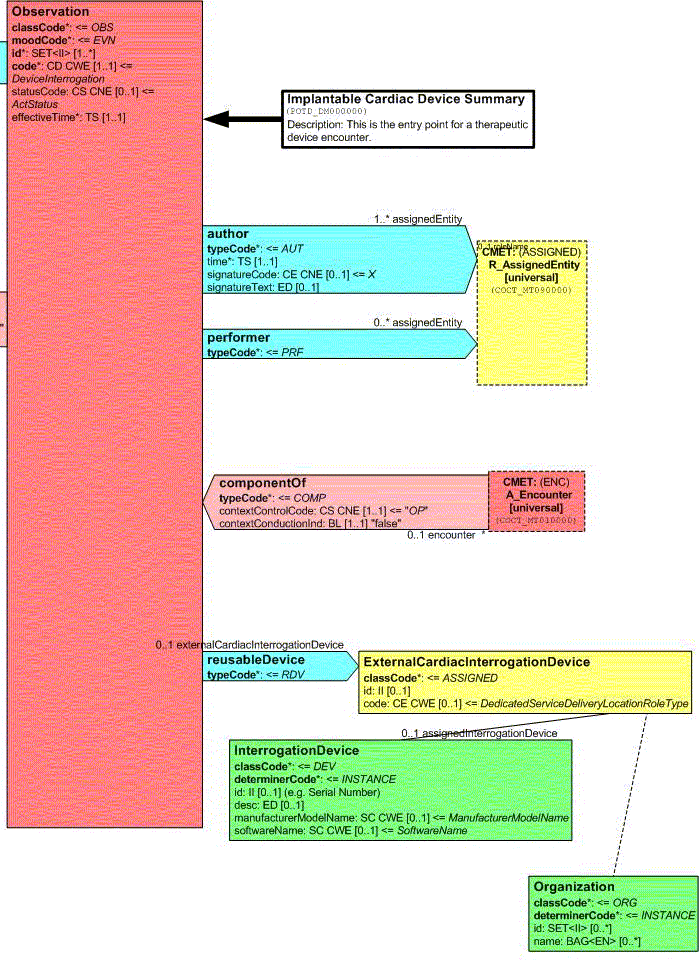 These Participations are:
These Participations are:
-
Author - A party that has responsibility for the information given in the Act and ownership of this Act. Note: The Author does not necessarily have to be present when the act occurs, but is accountable for the action through the power to delegate and the duty to review actions with the performing actor after the fact. The Author attributes are defined in the R_AssignedEntity CMET.
-
Performer - Identifies the role assisting in the Observation. The Performer attributes are defined in the R_AssignedEntity CMET.
-
Reusable Device - Identifies the device used to perform the interrogation that is being observed. The Reusable Device attributes are currently limited to the ExternalCardiacInterrogationDevice role, the interrogationDevice entity (player), and the Organization (scoper).
In addition, the Observation is a component of the A_Encounter CMET. This association enables information from this specific Observation event to be associated with other information from a patient encounter.
Conclusion
In simple terms, we have a message model for describing an Observation of an interrogator at a physical location who is performing an interrogation of a therapeutic medical device (bound to a patient) using identifiable interrogation equipment.
| Return to top of page |

