 HL7 CCPM, R1 HL7 Version 3 Standard: Common Product Model CMETs, DSTU Release 11 June 2011 |
Content Last Edited: 2011-06-14T14:26:17
Description of the services (actions) that a device performs, such as observation definitions for devices that produce observation results, but treatment/intervention actions (and usually in combination with observations) are also possible.
|
||||||||||
|
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
| Parent: | ProductKind (POCP_DM010000UV) |
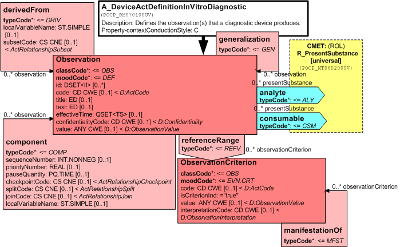
This is a definition of observation services that a diagnostic device performs. It focuses on an Observation in definition mood, where a code from a standard vocabulary (e.g. LOINC) can be provided to state what property the device measures. In addition, a specific link to the Substance may be given that is the analyte which the device detects. For example, if the device measures digoxin concentrations, the analyte is digoxin. Multiple observations (batteries) may be specified as a structure of sub-observations. If observations are calculated (e.g., anion gap from a blood gas analyzer) this can be specified with the derivedFrom relationships. Interpretation ranges of the results may be specified using the reference values and manifestation links.
| A_DeviceActDefinitionInVitroDiagnostic | POCP_HD070100UV |
| Parent: | ProductKind (POCP_DM010000UV) |
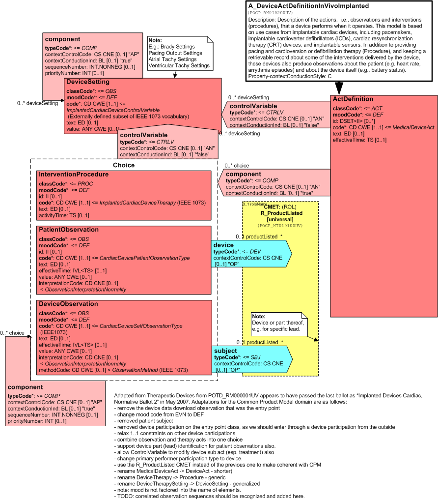
Description of the actions. i.e., observations and interventions (procedures), that a device performs when it operates. This model is based on use cases from implantable cardiac devices, including pacemakers, implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices, and implantable sensors. In addition to providing pacing and cardioversion or defibrillation therapy (Procedure), and keeping a retrievable record about some of the interventions delivered by the device, these devices also produce observations about the patient (e.g. heart rate, arrythmia episodes) and about the device itself (e.g., battery status).
Adapted from Therapeutic Devices from POTD_RM000001UV last balloted in the Implanted Devices Cardiac, Normative Ballot 2 - May 2007 but the final status is unclear (?).
| A_DeviceActDefinitionInVivoImplanted | POCP_HD070200UV01 |
| Parent: | ProductKind (POCP_DM010000UV) |
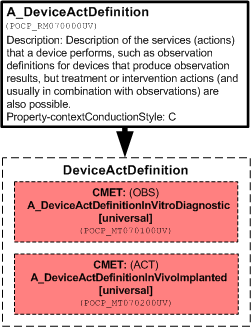
Description of the services (actions) that a device performs, such as observation definitions for devices that produce observation results, but treatment or intervention actions (and usually in combination with observations) are also possible.
The Device Act definition choice is the extension point to support in vitro diagnostic devices, implanted devices, and others. The device act is the service that the device itself performs. This is an observation for diagnostic devices and any other acts for other devices. For implanted pacemakers, for example, the device act would be a composite act consisting of sensing (observations) and pacing.
| A_DeviceActDefinition | POCP_HD070000UV01 |
|
||||||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
Defines the observation(s) that a diagnostic device produces.
| R_PresentSubstanceUniversal | POCP_MT080200UV01 |
| POCP_MT070100UV |
Description of the actions. i.e., observations and interventions (procedures), that a device performs when it operates. This model is based on use cases from implantable cardiac devices, including pacemakers, implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices, and implantable sensors. In addition to providing pacing and cardioversion or defibrillation therapy (Procedure), and keeping a retrievable record about some of the interventions delivered by the device, these devices also produce observations about the patient (e.g. heart rate, arrythmia episodes) and about the device itself (e.g., battery status).
| R_ProductListedUniversal | POCP_MT010100UV01 |
| A_DeviceActDefinitionInVivoImplanted | POCP_MT070200UV01 |
Description of the services (actions) that a device performs, such as observation definitions for devices that produce observation results, but treatment or intervention actions (and usually in combination with observations) are also possible.
| A_DeviceActDefinitionInVitroDiagnosticUniversal | POCP_MT070100UV |
| A_DeviceActDefinitionInVivoImplantedUniversal | POCP_MT070200UV01 |
| A_DeviceActDefinition | POCP_MT070000UV01 |
| Return to top of page |

