Regulated Reporting
 ANSI/HL7 V3 ICSRP1, R2-2012 HL7 Version 3 Standard: Pharmacovigilance - Individual Case Safety Report, Part 1: The Framework for Adverse Event Reporting, R2 (revise and partition ANSI/HL7 V3 RRCS, R1-2005) 1/31/2012 |
 ANSI/HL7 V3 ICSRP2, R2-2012 HL7 Version 3 Standard: Pharmacovigilance - Individual Case Safety Report, Part 2: Human Pharmaceutical Reporting Requirements for ICSR, R2 (revise and partition ANSI/HL7 V3 RRCS, R1-2005) 1/31/2012 |
| Responsible Group | Regulated Clinical Research Information Management Work Group HL7 |
| Primary Contributor | Austin Kreisler duz1@cdc.gov SAIC Consultant to the CDC |
| Primary Contributor | Steve Gitterman US Food and Drug Administration |
| Primary Contributor | Sandy Boyer Sandy Boyer Consulting |
| Primary Contributor | Gunther Schadow, M.D., PHD. Regenstrief Institute for Health Care |
| PHER Co-Chair | Rita Altamore rita.altamore@doh.wa.gov Washington State Department of Health |
| PHER Co-Chair | Jim Case jtcase@ucdavis.edu American Assoc. of Veterinary Lab Diagnosticians |
| Patietn Safety Co-Chair | Nick Halsey nick.halsey@ema.europa.eu European Medicines Agency |
| PHER Co-Chair | Daniel Pollock dap1@cdc.gov Centers for Disease Control and Prevention |
| Regulated Clinical Research Information Management Co-Chair | Edward Tripp edward.tripp@abbott.com Abbott Laboratories |
| Public Health and Emergency Response - Co-Chair | Alean Kirnak akirnak@swpartners.com Software Partners, LLC |
| Public Health and Emergency Response SIG Co-Chair | Michelle Williamson mwilliamson@cdc.gov CDC/National Center for Health Statistics (NCHS) |
| Public Health and Emergency Response SIG Co-Chair | Kristi Eckerson keckerson@cdc.gov Northrop Grumman |
| Patient Safety Co-Chair | Mead Walker dmead@comcast.net Independent Consultant, Health Data and Interoperability, Inc. |
| Patient Safety SIG Co-Chair | Stevens-Hawkins lise.stevens-hawkins@fda.hhs.gov US Food and Drug Administration |
| Participating Organization | ISO www.iso.org |
| Contributing Organization | International Conference on Harmonization www.ich.org |
| Modelling Facilitator | Nancy McQuillen nmcquill@dhs.ca.gov California Department of Health Services |
| Publishing Facilitator | Joginder Madra joginder.madra@gpinformatics.com Gordon Point Informatics Ltd. |
| Publishing Facilitator | Rob Savage hzv3@cdc.gov Northrup Grumman consultant to CDC |
| Primary Contributor | Patrick Loyd patrick.loyd@gpinformatics.com Gordon Point Informatics Ltd. |
| Primary Contributor | Mead Walker dmead@comcast.net Mead Walker Consulting |
| Primary Contributor | Julie M James MRPharmS julie_james@bluewaveinformatics.co.uk Blue Wave Informatics LLP |
| Contributor | Jason Rock jason.rock@globalsubmit.com Global Submit |
| Contributor | Gunther Schadow gschadow@regenstrief.org Indiana School of Medicine |
| PSSIG Co-Chair \ Primary Contributor | Rob Borotkanics RBorotka@ahrq.gov Agency for Healthcare Research and Quality |
| PSSIG Co-Chair \ Primary Contributor | Clive Flashman clive.flashman@npsa.nhs.uk UK National Patient Safety Agency |
| RCRIM Co-Chair | Randy Levin US Food and Drug Administration |
| RCRIM Co-Chair/Facilitator | Linda Quade Eli Lilly and Company |
| PSSIG Co-Chair | Lise Stevens StevensL@cber.FDA.gov US Food and Drug Administration |
| RCRIM Co-Chair | Barbara Tardiff Merck |
| Primary Contributor | Mead Walker Mead Walker Consulting |
HTML Generated: 2012-08-31T12:12:10
Content Last Edited: 2012-02-20T17:13:56
HL7® Version 3 Standard, © 2012 Health Level Seven® International All Rights Reserved.
HL7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U.S. Pat & TM Off.
Use of these materials is governed by HL7 International's IP Compliance Policy.
Table of Contents
Preface
i Notes to Readers
ii Message Design Element Navigation
1 Overview
1.1 Introduction & Scope
1.2 Domain Information Models
2 Individual Case Safety Reporting Topic
2.1 Introduction
2.2 Storyboards
2.3 Application Roles
2.4 Trigger Events
2.5 Refined Message Information Models
2.6 Hierarchical Message Descriptions
2.7 Interactions
3 Human Pharmaceutical Reporting Topic
3.1 Introduction
3.2 Storyboards
3.3 Application Roles
3.4 Trigger Events
3.5 Refined Message Information Models
3.6 Hierarchical Message Descriptions
3.7 Interactions
4 Quality Analysis Report Topic
5 CMETs Used by this Domain
6 Interactions Annex
6.1 By Application Role
6.2 By Trigger Event
6.3 By Message Type
7 Glossary
In Normative Edition 2011, the Public Health Reporting domain contains two "topics", as outlined below.
- Individual Case Safety Reporting Topics
- Individual Case Safety Report, Part 1; the framework for adverse event reporting, Release 1
- Individual Case Safety Report, Part 2; Human pharmaceutical reporting requirements for ICSR, Release 1
These materials arose out a Joint Initiative Council (JIC) harmonization effort between Technical Committee ISO/TC 215, Health Informatics, CEN TC 251, and the HL7 International Patient Safety Work Group.
|
||||||||
|
The domain includes messages and documents that are specifically designed to support reporting and investigation in the public health context. This scope includes:
- Communications within a health provider organization,
- Messages sent to public health regulatory agencies or other patient safety/quality improvement organizations from outside parties,
- Reporting among health care providers, manufacturers, and public health or patient safety/quality improvement organizations.
|
||||||||
|
Diagram
Introduction
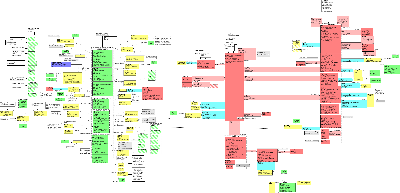
It should be noted that there is not currently a generally accepted Domain model for the PORR domain. Work is currently in progress involving PHER, Patient Safety, RCIRM, etc. to develop such a model.
The Public Health Reporting Domain Information Model captures the information to be exchanged about conditions or events that are to be subject to public health review or investigation. The model initially focused on the needs of the US Federal Government for HL7 messaging in the areas of disease surveillance and the reporting of adverse events, reactions or problems related to regulated products, as well as reporting by parties investigating incidents of public concern. However, the model is not limited to US based reporting, or to regulatory application - but has been generalized to be applicable across the HL7 community.
Overview
This model is an initial draft of a Public Health Domain Message Information Model (DMIM) that is expected to eventually cover
all topics in the PORR domain. The model shown above covers subject matter for the Investigation Request and Case Management
Topics, as well as the two principle public health cmets: E_PublicHealthEntity and A_PublicHealthStatement. Future versions
will extend the model to support Generic Incident (GIN) topic, Outbreak Management and Safety Reporting Management topics.
The source models for two additional cmets (A_coordinate and R_PublicHealthContactPerson will also be incorporated into this
dmim.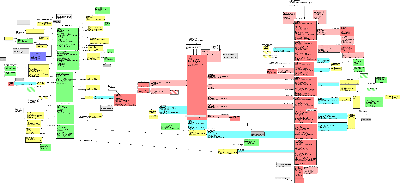
Attribute level descriptions have been prepared for most components of this model and are available in a separate document posted on the PHER Committee Website (http://hl7.org/Special/committees/pher/index.cfm).
Diagram
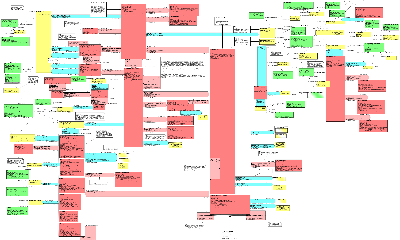
The Public Health Reporting Domain Information Model captures the information to be exchanged about conditions or events that are to be subject to public health review or investigation. The model initially focused on the needs of the US Federal Government for HL7 messaging in the areas of disease surveillance and the reporting of adverse events, reactions or problems related to regulated products, as well as reporting by parties investigating incidents of public concern. However, the model is not limited to US based reporting, or to regulatory application - but has been generalized to be applicable across the HL7 community.
The conditions or events that may be subject to review or investigation (i.e., the events that may trigger an investigation) are represented by the InvestigatedEventPackage class. These events that cause the investigation can be represented at any level of granularity (individual observations, cases, incidents, outbreaks, etc.), allowing specifications to be written with specificity appropriate to the target environment. In addition, triggering events can be disaggregated into more granular component events using the recursive component relationship. Subjects, assessment of relatedness to known interventions, outcomes, locations, reporters and any special qualifiers can be represented for each triggering event, and for each of its components.
The Intervention section of the model captures information about any interventions (procedures or substance administrations potentially applied to the subject of a triggering event) that might be implicated as causing or affecting the triggering events. The actual substances or devices involved in the interventions are described in the Product section of the model. Alternatively, these products may be investigated directly, absent an intervention in a subject, and so they are associated directly with the investigation as well. Other clinical information about the subjects of triggering events, or about individuals significantly related to these subjects, can be reported using the ClinicalStatement section of the model. Clinical information surrounding the interventions themselves can also be represented using this section.
The investigative process is represented in the InvestigationEvent class. Related to this class are known sources of information for the investigation, including identified reporters, related reports, and related documents (e.g. literature citations, primary source documents such as actual lab reports, discharge summaries, etc.). Information that is developed during the Investigation process, such as the assessment of overall seriousness, is also associated directly with the investigation.
It is important to understand that when this model is used to develop a reporting or notification specification, some particulars of such a report, e.g., report id, date/time of transmission, party transmitting the report, will not be represented by any of the elements of this model. They will, instead, be represented through the "trigger event" act contained in the "wrapper" model that preceeds the "payload" or report contents when the message is transmitted. It is the payload only that is derived from this DMIM
The reader should note that this DMIM may be subject to major revision in order to be harmonized with the RCRIM TC DMIM.
| Return to top of page |

