 ANSI/HL7 V3 RCMR, R1-2006 HL7 Version 3 Standard: Medical Records/Information Management, Release 1 11/3/2006 |
Content Last Edited: 2008-04-30T11:54:50
2.2 Storyboards
2.3 Application Roles
2.4 Trigger Events
2.5 Refined Message Information Models
2.6 Hierarchical Message Descriptions
2.7 Interactions
This topic defines the Document Management transaction set. It supports transmission of new or updated documents or information about their status(es). The trigger events and messages may be divided into two broad categories - one which describes the status of a document only and the other that describes the status and contains the document content itself. The conceptual purpose of document notification is to facilitate updating the receiving system(s) with information from the source system(s), typically dictation or transcription systems, to indicate that an electronic document has been created or altered. The document notification message can be attached to an entire document (i.e., transcribed document) or can be transmitted stand-alone. In either case, the document notification is transmitted in the form of an unsolicited update or in response to a record-oriented query. A document notification message can be created under a variety of circumstances such as when: 1) dictation has been completed; 2) a document has been transcribed; or 3) the status of a document has been changed, for example, when a document has been authenticated.
|
||||||||
|
For details on the interpretation of this section, see the storyboard discussion in the Version 3 Guide.
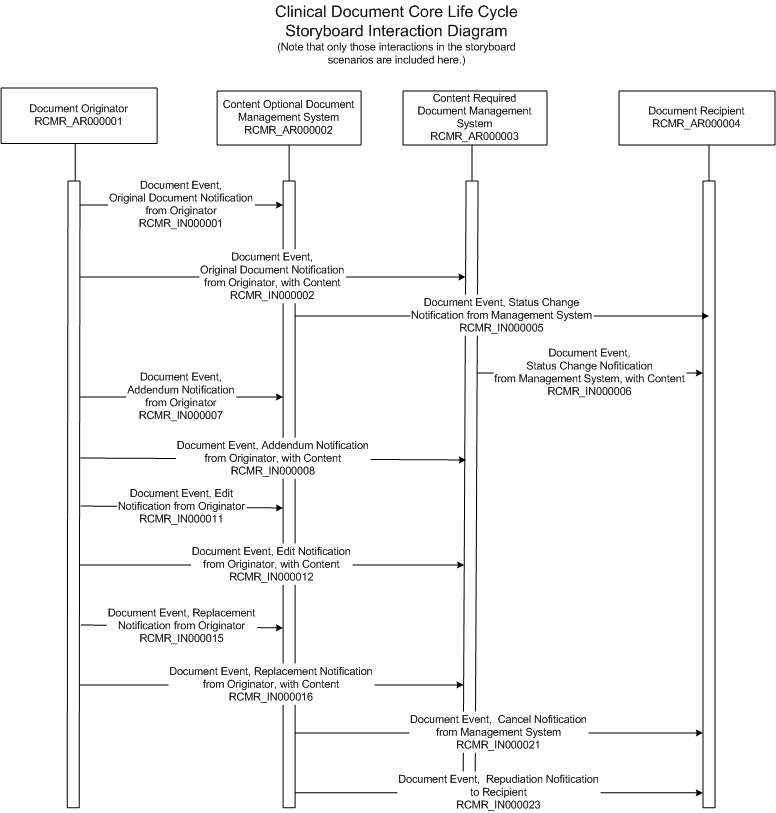
| Original Document | |
| Original Document with Content | |
| Document Status Change | |
| Document Status Change with Content | |
| Document Addendum | |
| Document Addendum with Content | |
| Document Edit Notification | |
| Document Edit Notification with Content | |
| Document Replacement | |
| Document Replacement with Content | |
| Document Cancel Notification |
A pathology resident performs a gross dissection examination of the tissue submitted from a surgical procedure to remove the gall bladder of a 37 year old female patient. The pathologist assigns a surgical case number to the study and dictates his observations into a central dictation system. This narrative is exposed to a transcriptionist who keys the text into a transcription manager application. This application generates a message from the Transcription Manager (RCMR_AR000001) to a specialized case of a document manager application called a surgical pathology system (RCMR_AR000002).
Since a complete surgical pathology exam contains microscopic findings and conclusions the Document Completion Status of the document is marked as "Incomplete".
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'new'
- ClinicalDocument.completionCode: 'incomplete'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
Later, another junior pathology resident performs the microscopic exam and dictates the remainder of the pathology report.
This incremental narrative is exposed to a transcriptionist who keys the additional text into the Transcription Manager application (RCMR_AR000001). This application generates a message from the Transcription Manager to a specialized case of a document manager called a surgical pathology system (RCMR_AR000002) with the document completion status of 'pre-authenticated'. Now that the content is presumed complete, the message includes the pathology examination notification as well as the findings.
Dependent on the business rules of the institution, this same message might be sent to a charting / clinical document repository application.
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'new'
- ClinicalDocument.completionCode: 'pre-authenticated'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
The junior resident who performed the microscopic exam now reviews the pathology exam finding and indicates in the document management system (RCMR_AR000002) that the finding(s) have been authenticated by him. In this case, the junior residents are by institutional policy not qualified to legally authenticate the results. As a policy, the institution distributes the documents when legally authenticated.
Now a message is generated from the document manager application to the charting or clinical document repository application (RCMR_AR000004) containing a document authentication status of "authenticated", but, as an institutional business rule, withholds the document content because the legal authentication is pending.
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'new'
- ClinicalDocument.completionCode: 'authenticated'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
Later a staff (board certified) pathologist "over-reads" the slides and the text of the report and agrees that the junior resident's findings were correct. This pathologist has sign-out authority. He thus legally authenticates this surgical pathology exam document.
Based on the business rules of the institution (as previously employed in the Document Status Change Notification example) a message is generated from the document manager application (RCMR_AR000003) to the charting / clinical document repository application (RCMR_AR000004) containing the full report content and an authentication status of "legally authenticated".
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'completed'
- ClinicalDocument.completionCode: 'legally authenticated'
- ClinicalDocument.availabilityTime: '20010718:0922'
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
During the initial gross dissection of the gall bladder tissue the prosector discovered a small, hard, 4mm brownish green inclusion that the pathology resident felt was consistent with a gall stone. This "stone" was removed and sent out to a reference laboratory for X-ray Crystallography analysis to confirm that the inclusion is indeed a gallstone and to determine the composition of the stone.
In preparing the specimen for submission to the reference laboratory, a requisition is filled out where the surgical case number assigned to the gallbladder surgical pathology exam is listed as the parent document ID together with the patient demographics and identifiers and the exam requested. The reference lab performs the steps of the specimen intake process for requested study with the original surgical case number being used as the parent document ID and their own reference laboratory assigned accession number being used as the report identifier.
The reference laboratory document manager application (RCMR_AR000001) generates a document addendum notification message to the referring hospital's surgical pathology document manager application (RCMR_AR000002) indicating the assigned document ID with a document completion status of "in progress".
Status field values (which apply to the addendum) contained in this message include:
- ClinicalDocument.statusCode: 'new'
- ClinicalDocument.completionCode: 'in progress'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'b456'
- ClinicalDocument.setId: 'b689'
- ClinicalDocument.versionNumber: '1'
- Relationship to parent document: 'APND'
- Parent ClinicalDocument.id: 'a123'
- Parent ClinicalDocument.setId: 'BB35'
The reference lab performs the analysis and confirms the presence of a gall stone and identifies the chemical composition of the crystals that make up the stone.
The text is verified by the X-ray Crystallography technician who then reviews the text online and authenticates its accuracy. In the reference laboratory the technician has, by institutional policy, the authority to sign out reports.
The reference laboratory document manager application (RCMR_AR000001) generates a document addendum notification and content message to the referring hospital's surgical pathology document manager application (RCMR_AR000003) indicating the assigned document ID with a document completion status of "legally authenticated".
Status field values (which apply to the addendum) contained in this message include:
- ClinicalDocument.statusCode: 'completed'
- ClinicalDocument.completionCode: 'legally authenticated'
- ClinicalDocument.availabilityTime: '20010721:1647
Identifier fields include:
- ClinicalDocument.id: 'b456'
- ClinicalDocument.setId: 'b689'
- ClinicalDocument.versionNumber: '1'
- Relationship to parent document: 'APND'
- Parent ClinicalDocument.id: 'a123'
Only documents that have not been released for patient care (i.e. have a statusCode of "new") can be edited. Once a document is "active" or "completed", it needs to be formally revised. The pathology resident has authenticated the original full text of the gall bladder surgical pathology exam, but because of institutional business rules, the document remains "new" until it is legally authenticated. The need for a change is discovered by the staff pathologist "over-reading" the case findings. The pathologist dictates the changes that will be subsequently keyed into the transcription manager application.
A message containing the notification of the intended edit is generated by the transcription manager application (RCMR_AR000001) and sent to the Document Manager application (RCMR_AR000002). Note that the ID and Set ID field values do not change during edits. Local business rules in each application domain dictate whether a document's version number is incremented with successive edits.
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'new'
- ClinicalDocument.completionCode: 'authenticated'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
The pathologist over-reading that surgical pathology exam identifies additional benign morphologic changes to the gall bladder and dictates the changes that are now keyed into the transcription manager application. Once the edited modification to the gall bladder surgical exam has been applied, a message will be generated.
A message containing the modified content is generated by the transcription manager application (RCMR_AR000001) and sent to Document Manager application (RCMR_AR000003). Because the content has not been released for patient care the Document Manager receiving the message over-writes the original content with the content supplied in this message.
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'new'
- ClinicalDocument.completionCode: 'authenticated'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
Only documents that have not been released for patient care (i.e. have a statusCode of "new") can be edited. Once a document is "active" or "completed", it needs to be formally revised. The pathology resident has authenticated the original full text of the gall bladder surgical pathology exam, and at the resident's institution, the policy is to make documents available for patient care before they are legally authenticated, so the document is advanced to "active" status. The need for a change is discovered by the staff pathologist "over-reading" the case findings. The pathologist dictates the changes that will be subsequently keyed into the transcription manager application.
A message containing the notification of the intended revision is generated by the transcription manager application (RCMR_AR000001) and sent to Document Manager application (RCMR_AR000002). In this case there is an earlier document available for patient care that has been found to be inaccurate.
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'active'
- ClinicalDocument.completionCode: 'authenticated'
- ClinicalDocument.availabilityTime: '20010723:1400
- ParentDocument.statusCode: 'obsolete'
Identifier fields include:
- ClinicalDocument.id: 'c266'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '2'
- Relationship to parent document: 'RPLC'
- Parent ClinicalDocument.id: 'a123'
- Parent ClinicalDocument.setId: 'BB35'
- Parent ClinicalDocument.statusCode: 'obsolete'
The pathologist over-reading that surgical pathology exam identifies additional benign morphologic changes to the gall bladder and dictates the changes that are now keyed into the transcription manager application. Once the revision to the gall bladder surgical exam has been applied, a message will be generated.
A message containing the notification and content of the change is generated as a replacement document by transcription manager application (RCMR_AR000001) and sent to Document Manager application (RCMR_AR000003).
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'active'
- ClinicalDocument.completionCode: 'authenticated'
- ClinicalDocument.availabilityTime: '20010723:1400'
Identifier fields include:
- ClinicalDocument.id: 'c266'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '2'
- Relationship to parent document: 'RPLC'
- Parent ClinicalDocument.id: 'a123'
- Parent ClinicalDocument.setId: 'BB35'
- Parent ClinicalDocument.statusCode: 'obsolete'
The gall bladder surgical pathology exam document was inadvertently assigned to the wrong patient. While an original document was generated it contained a status code value of 'new' (because it had not been made available for patient care). The document can be cancelled.
If a document has been made available for patient care, then this interaction cannot occur. Once any form of a document has been released for patient care it must be replaced or repudiated.
A message containing the cancellation is generated by the document management application (RCMR_AR000002) and sent to any and all previous recipients of Medical Document Management messages for this document (RCMR_AR000004).
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'cancelled'
- ClinicalDocument.completionCode: 'pre-authenticated'
- ClinicalDocument.availabilityTime: NULL
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
The gall bladder surgical pathology exam document was inadvertently assigned to the wrong patient, and the document was subsequently made available for patient care. An original document was generated and has acquired a status of 'active', so can no longer be canceled. Instead, the original document is repudiated, and its status is changed to 'nullified'.
Status field values contained in this message include:
- ClinicalDocument.statusCode: 'nullified'
- ClinicalDocument.completionCode: 'authenticated'
- ClinicalDocument.availabilityTime: '20010723:1400'
Identifier fields include:
- ClinicalDocument.id: 'a123'
- ClinicalDocument.setId: 'BB35'
- ClinicalDocument.versionNumber: '1'
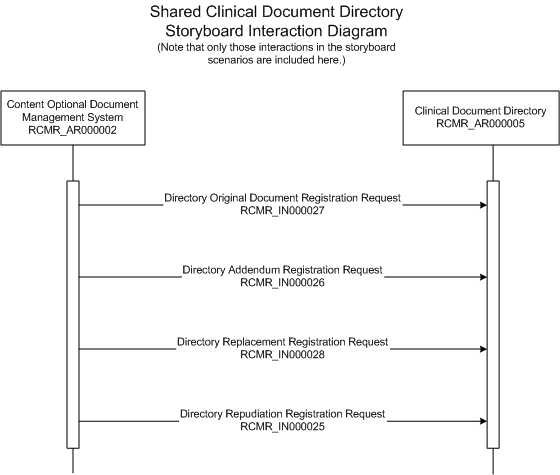
| Directory Original Document Registration Request | |
| Directory Addendum Registration Request | |
| Directory Replacement Registration Request | |
| Document Repudiation Notification From Originator |
A group of organizations have agreed to make their completed clinical documents available to one other (subject to appropriate security, confidentiality, and access control) through a shared Clinical Document Directory. Organization One's document management system acquires a new legally authenticated clinical document, and sends a notification to the Clinical Document Directory. The Clinical Document Directory maintains a directory of completed clinical documents that it has been made aware of, without the actual document contents.
NOTE: Appropriate security, confidentiality, and access control is subject to the rules governing the particular directory, and is outside the scope of this specification. Also outside the scope is the creation and/or maintenance of any type of Master Patient Index.
Directory Addendum Registration Request(RCMR_IN000026)Organization One's document management system acquires a legally authenticated addendum to a clinical document for which a notification message has previously been sent to a shared Clinical Document Directory, and sends a document addendum notification to the directory.
Directory Replacement Registration Request(RCMR_IN000028)Organization One's document management system acquires a replacement to a clinical document for which a notification message has previously been sent to a shared Clinical Document Directory, and sends a document replacement notification to the directory.
Directory Repudiation Registration Request(RCMR_IN000025)Organization One's document management system learns that a completed document for which a notification message has previously been sent to a shared Clinical Document Directory was issued with the wrong patient name, and sends a document repudiation notification to the directory.
|
||||||||||||||
|
For details on the interpretation of this section, see the discussion of application roles and their relationships in the Version 3 Guide.
An application role that maintains a directory of completed clinical documents that are housed in the document management systems of contributing organizations. Can serve as a document requestor by generating a document query message.
NOTE: Appropriate security, confidentiality, and access control is subject to the rules governing the particular directory, and is outside the scope of this specification. Also outside the scope is the creation and/or maintenance of any type of Master Patient Index within the directory.
An application role that tracks the status of documents including revisions, addenda, and authentications; provides access to and preserves documents in permanent and secure storage. Can receive document status update messages that do not also contain the associated document. Can serve as a document requestor by generating a document query message.
An application role that tracks the status of documents including revisions, addenda, and authentications; and provides access to and preserves documents in permanent and secure storage. Requires that document status update messages also transmit the associated document. Can serve as a document requestor by generating a document query message.
|
||||||||||||||||||
|
For details on the interpretation of this section, see the discussion of trigger events in the Version 3 Guide.
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
| Document (RCMR_RM000050UV) | |
| Document (RCMR_RM000050UV) |
This is a notification of an addendum to a document. For instance, an author dictates additional information as an addendum to a previously transcribed document. A new document is transcribed. This addendum has its own new unique document ID that is linked to the original document via the parent ID. Addendum document notification is transmitted. This creates a composite document.
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
| Document (RCMR_RM000050UV) |
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
This is a notification of an edit to a document. For instance, errors which need to be corrected, are discovered in a document. The original document is edited, and an edit notification is sent. Note that the only valid use of this trigger event is for documents that have not yet been made available for patient care.
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
| Document (RCMR_RM000050UV) | |
| Document (RCMR_RM000050UV) |
This is a notification of the creation of a document. There are multiple approaches by which systems become aware of documents: (1) A document is dictated and chart tracking system is notified that it has been dictated and is awaiting transcription; (2) Dictation is transcribed and chart tracking system is notified that the document exists and requires authentication; (3) A provider orders a series of three X-rays. The radiologist dictates a single document which covers all three orders; (4) A system automatically (time-based or demand-based) generates a clinical report from a constellation of existing data.
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
| Document (RCMR_RM000050UV) | |
| Document (RCMR_RM000050UV) |
This is a notification of replacement to a document. For instance, errors discovered in a document are corrected. The original document is replaced with the revised document. The replacement document has its own new unique document ID that is linked to the original document via the parent ID. The status of the original document is changed to obsolete but the original document should be retained in the system for historical reference. Document replacement notification is sent. Note that this trigger event is used when the original document has been made available for patient care.
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
| Document (RCMR_RM000050UV) | |
| Document (RCMR_RM000050UV) | |
| Document (RCMR_RM000050UV) | |
| Document (RCMR_RM000050UV) |
This is a notification of a change in a status of a document. For instance, a document is authenticated. Notification is sent to the chart tracking system and is used to up-date the document status from pre-authenticated to authenticated or legally authenticated. A change in any of the following characteristics would cause a message to be sent: (1) completionCode; (2) confidentialityCode; (3) availabilityTime; (4) storageCode.
| Type: | User request |
| State Transition: | Document (RCMR_RM000050UV) |
This is a notification of a cancellation of a document. This trigger event should be used only for an original document that has not been made available for patient care. (When a document has been made available for patient care, the process should be to replace the original document, which then becomes obsolete.) For instance, when the author dictated a document, the wrong patient identification was given. Upon review of the transcribed document, the provider notes the error and the document is cancelled.
|
||||||
|
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
| Parent: | Medical Records (RCMR_DM000050UV) |
The Message Model is a subset of the Domain Model, thus all descriptions of the model are found under the discussion of the Domain Model. This section enumerates the changes that are made in going from the Domain Model to the Message Model:
- Document Attributes
- There are no changes from the Domain Model
- Document Relationships
- There are no changes from the Domain Model
- Document Participants
- There are no changes from the Domain Model
| Medical Records HMD | RCMR_HD000050UV02 |
|
||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
The Medical Records and Structured Documents specifications derive from a common model.
Message RCMR_MT000001 sends document metadata but not the document. Message RCMR_MT000002 sends both document metadata and the document. Registry interactions only use RCMR_MT000001, and can use the HL7 V3 Refinement and Localization process to constrain the message (e.g. to comply with local privacy and confidentiality regulations).
| R_Patient | COCT_MT050000UV01 |
| R_AssignedPerson | COCT_MT090100UV01 |
| E_Organization | COCT_MT150000UV02 |
| Document Event | RCMR_MT000001UV02 | |
| Document Event, with Content | RCMR_MT000002UV02 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
For details on the interpretation of this section, see the definition of Interactions in the Version 3 Guide.
| Trigger Event | Document Addendum Notification | RCMR_TE000506UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Addendum Notification | RCMR_TE000506UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Addendum Notification | RCMR_TE000506UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Required Document Management System | RCMR_AR000003UV02 |
| Trigger Event | Document Addendum Notification | RCMR_TE000506UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Optional Document Management System | RCMR_AR000002UV02 |
| Trigger Event | Document Edit Notification | RCMR_TE000708UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Edit Notification | RCMR_TE000708UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Edit Notification | RCMR_TE000708UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Required Document Management System | RCMR_AR000003UV02 |
| Trigger Event | Document Edit Notification | RCMR_TE000708UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Optional Document Management System | RCMR_AR000002UV02 |
Registry interactions only use message type RCMR_MT000001, and can use the HL7 V3 Refinement and Localization process to constrain the message (e.g. to comply with local privacy and confidentiality regulations).
| Trigger Event | Original Document Notification | RCMR_TE000102UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Master File / Reg. Notif Control Act, Act Subject | MFMI_MT700702UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Clinical Document Directory | RCMR_AR000005UV02 |
| Trigger Event | Original Document Notification | RCMR_TE000102UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Original Document Notification | RCMR_TE000102UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Original Document Notification | RCMR_TE000102UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Required Document Management System | RCMR_AR000003UV02 |
| Trigger Event | Original Document Notification | RCMR_TE000102UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Optional Document Management System | RCMR_AR000002UV02 |
| Trigger Event | Document Replacement Notification | RCMR_TE000910UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Replacement Notification | RCMR_TE000910UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Replacement Notification | RCMR_TE000910UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Required Document Management System | RCMR_AR000003UV02 |
| Trigger Event | Document Replacement Notification | RCMR_TE000910UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Optional Document Management System | RCMR_AR000002UV02 |
Registry interactions only use message type RCMR_MT000001, and can use the HL7 V3 Refinement and Localization process to constrain the message (e.g. to comply with local privacy and confidentiality regulations).
| Trigger Event | Document Replacement Notification | RCMR_TE000910UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Master File / Reg. Notif Control Act, Act Subject | MFMI_MT700702UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Clinical Document Directory | RCMR_AR000005UV02 |
| Trigger Event | Document Repudiation Notification | RCMR_TE000014UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Content Required Document Management System | RCMR_AR000003UV02 |
Registry interactions only use message type RCMR_MT000001, and can use the HL7 V3 Refinement and Localization process to constrain the message (e.g. to comply with local privacy and confidentiality regulations).
| Trigger Event | Document Repudiation Notification | RCMR_TE000014UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Master File / Reg. Notif Control Act, Act Subject | MFMI_MT700702UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Clinical Document Directory | RCMR_AR000005UV02 |
| Trigger Event | Document Repudiation Notification | RCMR_TE000014UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Status Change Notification | RCMR_TE000304UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Status Change Notification | RCMR_TE000304UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Cancel Notification | RCMR_TE000011UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Cancel Notification | RCMR_TE000011UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Receiver | Document Recipient | RCMR_AR000004UV02 |
| Trigger Event | Document Cancel Notification | RCMR_TE000011UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event, with Content | RCMR_MT000002UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Required Document Management System | RCMR_AR000003UV02 |
| Trigger Event | Document Cancel Notification | RCMR_TE000011UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Trigger Event Control Act | MCAI_MT700201UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Document Originator | RCMR_AR000001UV02 |
| Receiver | Content Optional Document Management System | RCMR_AR000002UV02 |
Registry interactions only use message type RCMR_MT000001, and can use the HL7 V3 Refinement and Localization process to constrain the message (e.g. to comply with local privacy and confidentiality regulations).
| Trigger Event | Document Addendum Notification | RCMR_TE000506UV02 |
| Transmission Wrapper | Send Message Payload | MCCI_MT000100UV01 |
| Control Act Wrapper | Master File / Reg. Notif Control Act, Act Subject | MFMI_MT700702UV01 |
| Message Type | Document Event | RCMR_MT000001UV02 |
| Sender | Content Optional Document Management System | RCMR_AR000002UV02 |
| Sender | Content Required Document Management System | RCMR_AR000003UV02 |
| Receiver | Clinical Document Directory | RCMR_AR000005UV02 |
| Return to top of page |


