Clinical Decision Support
 ANSI/HL7 V3 INFOB, R1-2010 HL7 Version 3 Standard: Context-Aware Retrieval Application (Infobutton); Knowledge Request, Release 1 7/21/2010 |
 HL7 V3 IG INFOBUTTON, R2 INFORM 2008JUN HL7 Version 3 Implementation Guide: URL-Based Implementations of the Context-Aware Retrieval Application (Infobutton) Domain, Release 2 June 2008 |
 HL7 V3 IG INFOBUTTON, R3 INFORM 2010DEC HL7 Version 3 Implementation Guide: URL-Based Implementations of the Context-Aware Retrieval Application (Infobutton)Domain, Release 3 December 2010 |
 HL7 V3 IG SOA KM INFOBUTTON, DSTU R1 2011MAR HL7 Version 3 Implementation Guide: URL-Based Implementations of the Context-Aware Retrieval Application (Infobutton)Domain, Release 3 March 2011 |
 HL7 V3 OSP, DSTU R1 2012SEP HL7 Version 3 Standard: Order Set Publication, Release 1 September 2012 |
| Responsible Group | Clinical Decision Support Work Group HL7 |
| Co-chair | Robert Greenes, MD, PhD Partners Healthcare/Brigham & Women's Hospital |
| Lead Editor & Co-Chair | Guilherme Del Fiol, MD, PhD guilherme.delfiol@duke.edu Duke University |
| Lead editor | James R. Campbell, MD University of Nebraska |
| Co-chair | Robert A. Jenders, MD, MS Cedars-Sinai Medical Center/University of California, Los Angeles |
| Co-chair | Robert Jenders, MD, MS jenders@ucla.edu Cedars-Sinai Medical Center/UCLA |
| Co-chair | Kensaku Kawamoto, MD, PhD kawam001@mc.duke.edu Duke University |
| CDS co-chair and contributor | Guilherme Del Fiol, MD, PhD University of Utah |
| Co-chair | R. Matthew Sailors, PhD University of Texas, Houston |
| Co-chair and M&M Facilitator | Craig Parker, MD craigparkermd@gmail.com Arizona State University |
| Editor | Guilherme Del Fiol, MD, MS Intermountain Healthcare, Salt Lake City |
| Co-chair | Robert Greenes, MD greenes@asu.edu Arizona State University |
| Editor/M&M Facilitator/Co-chair | Craig Parker, MD, MS Intermountain Healthcare, Salt Lake City |
| Contributor | Howard Strasberg, MD, MS Howard.Strasberg@wolterskluwer.com Wolters Kluwer Health |
| Contributor | James J Cimino, MD ciminoj@cc.nih.gov Clinical Center, National Institutes of Health |
| Contributor | James Cimino, MD Columbia University, New York |
| Contributor | Robert Dolin, MD Kaiser Permanente |
| Contributor | Thom Kuhn American College of Physicians |
| Contributor | Saverio Maviglia, MD, PhD Partners Healthcare, Boston |
| Contributor | Roberto Rocha, MD, PhD University of Utah, Salt Lake City |
| Contributor | Howard Strasberg, MD WoltersKluwer Health |
| Contributor | Bob Dolin, MD bobdolin@gmail.com Semantically Yours |
| Contributor | Saverio M Maviglia, MD, MSc smaviglia@partners.org Partners Healthcare |
| Contributor | Clayton Curtis, MD Clayton.Curtis@va.gov Veterans Health Administration |
| Contributor | Roberto Rocha, MD, PhD Partners Healthcare |
| Contributor | Thom Kuhn tkuhn@acponline.org American College of Physicians |
| Contributor | Nick Ackerson Thomson Reuters Health |
| Contributor | Aziz Boxwala Partners Healthcare, Boston, MA |
| Contributor | Andrew Gelsey Tomson Reuters Health |
| Contributor | Penny Hernandez Thomson Healthcare, Micromedex |
| Contributor | Nathan Hulse PhD Intermountain Healthcare, Salt Lake City, UT |
| Contributor | Victor Lee Zynx Health |
| Contributor | Patrick Lloyd |
| Contributor | James McClay University of Nebraska |
| Contributor | Jerry Osheroff MD Thomson Reuters Health |
| Contributor | John Robotham Up To Date |
| Contributor | Dan Russler |
| Contributor | Michael Schenk Elsevier/Informed Decisions LLC; Tampa, FL |
| Contributor | Pamela Sessions Elsevier/Informed Decisions LLC; Tampa, FL |
HTML Generated: 2012-08-31T12:09:02
Content Last Edited: 2012-08-14T11:54:42
HL7® Version 3 Standard, © 2010 Health Level Seven® International All Rights Reserved.
HL7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U.S. Pat & TM Off.
Use of these materials is governed by HL7 International's IP Compliance Policy.
Table of Contents
Preface
i Notes to Readers
ii Message Design Element Navigation
1 Overview
1.1 Introduction & Scope
1.2 Domain Analysis Model
1.3 Domain Information Models
2 Context-aware Knowledge Retrieval (Infobutton) Topic
2.1 Introduction
2.2 Storyboards
2.3 Application Roles
2.4 Trigger Events
2.5 Refined Message Information Models
2.6 Hierarchical Message Descriptions
2.7 Interactions
2.A Implementation Guide for Context-aware Knowledge Retrieval (Infobutton)
3 Order Sets Topic
3.1 Introduction
3.2 Storyboards
3.3 Application Roles
3.4 Trigger Events
3.5 Refined Message Information Models
3.6 Hierarchical Message Descriptions
3.7 Interactions
4 Quality Analysis Report Topic
5 Interactions Annex
5.1 By Application Role
5.2 By Trigger Event
5.3 By Message Type
6 Glossary
In this publication, the Clinical Decision Support domain contains multiple "topics", as outlined below:
- Context-aware Information Retrieval Topic (Infobutton): Normative document.
- Infobutton URL-Based Implementation Guide: Informative document.
- Order Sets: Draft Standard for Trial Use.
The goal of the Infobutton standard is to facilitate the integration of knowledge resources into clinical information systems in order to reduce barriers to the access of knowledge resources at the point of decision making, helping clinicians and patients meet their information needs. The specification consists of a standard mechanism for clinical information systems to request context-specific knowledge from knowledge resources (knowledge request). This specification was approved as an HL7 Normative standard following its successful ballot during the HL7 May 2010 ballot cycle. A preliminary version of this specification was approved as a DSTU in January 2008. The specification was widely validated and adopted by key stakeholders in the US and abroad during this trial period. The draft standard was selected by HITSP as the preferred standard for the retrieval of medical knowledge (HITSP/T81).
Although both the knowledge request and knowledge response interactions are described in this specification, the specification only includes the knowledge request information model.
The Order Sets topic describes a standard for sharing of order set structure and content between collaborating institutions. It offers a means to publish order set and care plan content but does not propose to specify order session processing or order communication within the healthcare enterprise.
|
||||||
|
The Context-aware Knowledge Retrieval specification also includes and accompanying informative document entitled Context-aware Knowledge retrieval (Infobutton), knowledge request. URL-Based Implementation Guide. This implementation guide provides a specification for URL-based implementations of the Context-aware knowledge retrieval (Infobutton) standard. The intent of this specification is to support the majority of implementations that offer URLs as the primary or exclusive communication protocol. The ultimate goal is to enable a future, stepwise transition from a URL-based to a Services-Oriented approach.
- Based on affirmative comments received during the May 2010 ballot cycle, the term "electronic health record" was replaced with the broader term "clinical information system" throughout the specification.
Order Sets Domain Analysis Model diagram

| RIM attribute | HL7 Data type | OS Use case | |
|---|---|---|---|
| Order set header | |||
| Order set unique identifier | |||
| Order set key | OrderSetDocument.id | II | I |
| OS Version | OrderSetDocument.versionNumber | INT | I |
| ============================ | |||
| Order set title | OrderSetDocument.title | String | I |
| Order set description | OrderSetDocument.summary.Description.text | String | I |
| Order set use case scenario | OrderSetBody.reason.UseCase.text | ED | I |
| Order Set Acuity | OrderSetBody.precondition.AcuityCriterion.code | CD CWE | I |
| Order Set Population | OrderSetBody.precondition.CriterionGroup.code; value | CD CWE;ANY | I |
| Order Set Owner | OrderSetDocument.custodian.Organization.id | II | I |
| Order Set author | OrderSetDocument.author.DocumentAuthor.Person.name | PN | I |
| Order Set author (creation) date | OrderSetDocument.effectiveTime[early bound] | TS | I |
| Order Set editor | OrderSetDocument.informant.AssignedEditor.Person.name | PN | I |
| Order Set edit date | OrderSetDocument.informant.time | TS | I |
| Order Set validator | OrderSetDocument.authenticator.Organization or Person.name | ST or PN | I |
| Order Set validation date | OrderSetDocument.authenticator.time | TS | I |
| Order Set archive date | OrderSetDocument.effectiveTime[late bound] | TS | I |
| Order Set Replaces | OrderSetDocument.predecessor.ParentDocument.id | II | I |
| Order Set Resource link | OrderSetDocument.support.OrderDocumentExternalReference.text | ED | III |
| Order Set Is Personal | OrderSetDocument.custodian.Person.name | PN | I |
| Order Set Problem code | OrderSetBody.precondition.ConcernCriterion.code | CD CWE | III |
| Order Set Session code | OrderSetBody.precondition.SessionCriterion.code | CD CWE | III |
| Milestone name | OrderSetDocument.appendage.Milestone.title | ST | I |
| Milestone creation date | OrderSetDocument.appendage.Milestone.effectiveTime[earlyBound] | TS | I |
| Milestone target date | OrderSetDocument.appendage.Milestone.goal.TargetCompletion.value | TS | I |
| Milestone actual date | OrderSet Document.appendage.Milestone.effectiveTime[lateBound] | TS | I |
| Order set Body | |||
| Order Section alert | OrderSetSection.subject.OrderSetSectionAlert.text | ED | II |
| Order Item alert | ActChoice.subject.OrderItemAlert.text | ED | II |
| Order display group | OrderSetSection.title | ST | II |
| Order Set boolean collective | OrderSetSection.component.conjunctionCode | CS CNE | II |
| Order item Pre-selection flag | ActChoice.trigger.SelectionStatus.code | CD CNE: "AND", "OR", "XOR" | II |
| Order Item Resource link | ActChoice.support.OrderItemExternalReference.text | ED | II |
| Strength of recommendation | ActChoice.referenceRange.StrengthofRecomendation.code; value | CD CWE; ANY | III |
| Order Item Note | ActChoice.reference.Note.text | ST | II |
| Order Act Definition | |||
| Full text order item | ActChoice.text | ED | II |
| Order sequence number | ActChoice.id | II | II |
| classCode | ActChoice.classCode | OBS,SBADM,SPLY,PROC | III |
| moodCode | ActChoice.moodCode | RQO,EVN,GOL | III |
| Order item service code | ActChoice.code | CD CWE | III |
| Order/goal/observation stop and start time | ActChoice.effectiveTime | GTS | III |
| Order priority | ActChoice.priorityCode | CE CWE | III |
| Order repetition | ActChoice.repeatNumber | IVL<INT> | III |
| Observation value | ObservationEvent.value | ANY CWE | III |
| Goal assertion value | GoalAssertion.value | ANY CWE | III |
| Medication or Treatment | SubstanceAdministrationRequest.product.LabeledDrug.code | CE CWE | III |
| Medication route | SubstanceAdministrationRequest.routeCode | CE CWE | III |
| Medication dose | SubstanceAdministrationRequest.doseQuantity | IVL<PQ> | III |
| Medication rate | SubstanceAdministrationRequest.rateQuantity | IVL<PQ> | III |
| Supply | SupplyRequest.product.ManufacturedProduct.Material.code | CE CWE | III |
| Procedure method | ProcedureRequest.methodCode | CE CWE | III |
| Procedure site | ProcedureRequest.approachSiteCode | SET<CD> | III |
Diagram
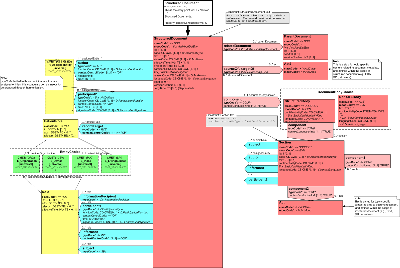
REDS_DM000001UV is a copy reference to the Structured Documents Domain Message Information Model - POCD_RM000001.UV - reproduced here for ease of review
Clinical decision Support collaborated with Structured Documents, Medical Records, Structured Product Labeling and Patient Care to develop the domain model for a structured document in order to harmonize model development between the technical committees. This DMIM reflects the product of that collaboration and is the more general supertype model of the the Order Sets Document RMIM REDS_RM020001UV included in this ballot.
| Return to top of page |

