 HL7 CCPM, R1 HL7 Version 3 Standard: Common Product Model CMETs, DSTU Release 11 June 2011 |
Content Last Edited: 2011-06-14T14:26:17
Generic events that may be recorded on product instances. This includes production, transportation, testing, maintenance, but typically excludes the actual clinical use (e.g., supply, administration, adverse events.)
|
||||||
|
For details on the interpretation of this section, see the description of RMIMs in the Version 3 Guide.
| Parent: | ProductKind (POCP_DM010000UV) |
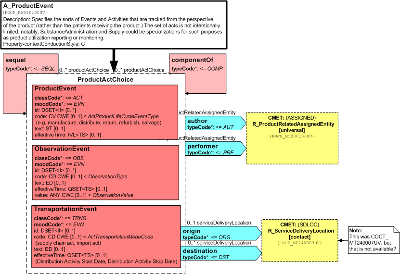
Specifies the sorts of Events and Activities that are tracked from the perspective of the product (rather than the patients receiving the product.) The set of acts is not intentionally limited, notably, SubstanceAdministration and Supply could be specializations for such purposes as product utilization reporting or monitoring.
| A_ProductEvent | POCP_HD040100UV01 |
|
||||||
|
For details on the interpretation of this section, see the description of HMDs in the Version 3 Guide.
Specifies the sorts of Events and Activities that are tracked from the perspective of the product (rather than the patients receiving the product.) The set of acts is not intentionally limited, notably, SubstanceAdministration and Supply could be specializations for such purposes as product utilization reporting or monitoring.
| R_ServiceDeliveryLocationContact | COCT_MT240003UV02 |
| R_ProductRelatedAssignedEntityUniversal | POCP_MT030100UV01 |
| A_ProductEvent | POCP_MT040100UV01 |
| Return to top of page |

